Making organic chemistry more green

Melissa D'Amaral
What comes to mind when you think of efforts to be more sustainable? Reducing single-use plastics and packaging? Electric vehicles? Renewable sources of energy? In reality, there are innumerable green initiatives underway that, while lesser known, are each important for achieving a greener future.
One such effort is taking place within the synthetic organic chemistry community. In 2018, the American Chemical Society’s Green Chemistry Institute Pharmaceutical Roundtable (GCIPR) listed “sustainable direct amide bond formation” as one of its ten key green chemistry research areas.
In organic chemistry, the amide bond is a common functional group. It makes up proteins, is found in many synthetic materials (such as nylon), and is present in about 25% of mainstream drugs on the market, in addition to many commercially available products.
The most common method for making amide bonds uses coupling reagents that facilitate the reaction by activating a carboxylic acid so it can more easily react with an amine. The problem is that these reagents can be difficult and unsafe to work with, which has made the search for greener and safer methods for making amides an area of interest for organic chemists.
Working with her supervisor, Dr. Marc Adler, first year Molecular Science master’s candidate Melissa D’Amaral is contributing to this effort. She recently published a paper called “Efficient and accessible silane-mediated direct amide coupling of carboxylic acids and amines” (external link) in the Royal Society of Chemistry’s journal Green Chemistry.
“Our work focuses on the use of organosilanes, silicon-based chemicals that have a carbon bonded to silicon,” says D’Amaral. “In terms of greener chemistry, they are attractive because silicon is naturally abundant and silanes are relatively inert, making them safer to work with and easier to store. They are also widely commercially available and used in a variety of reactions in organic chemistry.”
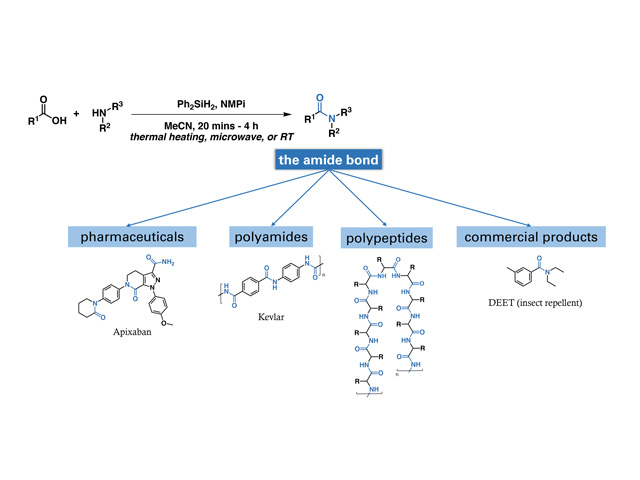
General method for amide synthesis developed in our lab, and various molecules that contain amides, such as top-selling small molecule pharmaceuticals, polymers, polypeptides, and store-bought products.
In the past five years, many research groups have demonstrated the use of organosilanes in amide synthesis and developed relatively efficient methods. But certain drawbacks have limited widespread adoption, specifically that the reaction requires extended heating and the absence of air or water.
“Our research found that by using an additive to inhibit salt formation, the amides can be synthesized in good yield without the typical drawbacks,” says D’Amaral. “Our method is practical and advantageous because the reaction is done without energy- and time-consuming exclusion of air or water and can happen quickly and occur at room temperature.”
The new method offers an alternative route for those seeking to make small molecules that contain amide bonds in an efficient and operationally simple way. The research also determined optimized reaction conditions for best yields and conducted a substrate scope to look at the range of amides that the method could produce.
Going forward, D’Amaral will continue to research the use of organosilanes for direct amide bond formation. She will explore greener and more efficient methodologies by designing silanes to catalytically enable the reaction between a carboxylic acid and an amine.

Funding of this research was provided by a Ryerson University Undergraduate Research Opportunity (URO), and three sources from the Natural Sciences and Engineering Research Council of Canada (NSERC): a Discovery Grant, a Canada Graduate Scholarship—Masters (CGS-M), and an Undergraduate Student Research Award (USRA).