Research Areas

Microfluidic systems that generate water droplets surrounded by oil (so-called, water-in-oil droplets) have been used for more than a decade to realize important applications such as drug discovery, single cell analysis, and cellular delivery. However, a well-known fundamental challenge of water-in-oil droplets in discovery and delivery applications is the need for extensive post-processing due to the toxicity of the oil phase, making an oil-free, biocompatible droplet microfluidics platform highly desirable.
At LoFFI, we are developing a suite of technologies that utilize all-aqueous water-in-water droplet microfluidics. The distinct advantage of microfluidic water-in-water droplets, as opposed to the classical microfluidic water-in-oil droplets, is the absence of the toxic oil phase, making water-in-water droplets all biocompatible.
Such water-in-water droplets are composed of aqueous two-phase system (ATPS) fluids, which form due to the mixture of high concentrations of at least two incompatible polymers in water, and subsequent phase separation. In recent years, we have been able to devise innovative approaches to passively generate water-in-water droplets with microfluidics, apply the droplets to encapsulate and controllably release cells, manipulate the droplets magnetically, stabilize the droplets with particles, polymerize the droplets into spiky particles, among other things.
Our group now aims to apply our expertise, in collaboration with biotechnology companies and hospital partners, to translate this technology towards applications in drug delivery and recapitulate in vivo biological phenomena, such as intracellular phase separation.
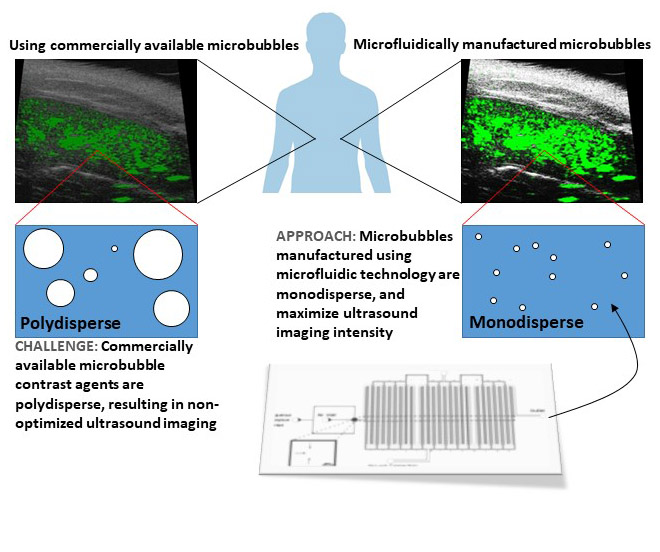
Lipid-stabilized microbubbles in the diameter range of 1 - 10 μm are used in clinical biomedical ultrasound as enhanced contrast agents and increasingly as therapeutic agents. Due to the difference in density and compressibility between the gas core of microbubbles and the surrounding blood and tissue, microbubbles produce strong echoes under an applied ultrasound, leading to significantly improved accuracy of detecting many diseases compared to ultrasound diagnostics without microbubbles.
Despite the utility of microbubbles in ultrasound imaging and therapeutics, commercially available microbubbles have large size distributions, which result in sub-optimal imaging conditions and therapeutic effects. This issue of polydisperse microbubbles is elegantly resolved by using microfluidics to make the bubbles. With microfluidic approaches, highly monodisperse microbubbles can be produced, often with polydispersity indices of < 1 %, and with excellent control of microbubble size.
At LoFFI, we have developed approaches to controllably shrink microbubbles in microfluidic systems, and mathematically model the shrinking process. Our group is now working on generating sub 1 μm diameter bubbles, and increasing the throughput of the bubble generation process.